Shubham Mondal awarded Rackham Predoc to support research on III-Nitride semiconductors for next generation electronics

Shubham Mondal, PhD student in ECE, was awarded a Rackham Predoctoral Fellowship for his research into III-Nitride semiconductors for next generation electronics and photonics.
III-Nitride semiconductors have gained significant attention in recent years due to their qualities of having a wide and tunable direct bandgap coupled with strong spontaneous and piezoelectric polarizations, qualities that make them suitable for next-gen power electronics, optoelectronics, piezo electronics, and integrated photonics. More recently, the incorporation of rare earth elements, e.g., Sc and Y, can transform conventional III-nitrides to be ferroelectric with significantly enhanced piezoelectric and nonlinear optical properties. The research is expected to drive innovation in the fields of in-memory computing, edge intelligence, quantum photonics, as well as high frequency, high power, and high temperature electronics.
“Polarization makes III-nitrides unique, and the ability to precisely control the polarization makes III-Nitrides attractive for UV-optoelectronics (LEDs, lasers, etc.) and power device (FETs, HEMTs, etc.) applications,” explained Mondal. “Going one step further, the ability to controllably switch the polarization, as is the case in recently discovered ferroelectric III-Nitrides, opens up a new dimension in III-nitrides for applications in the previously unexplored fields of data centric computing and edge intelligent devices.”
Mondal has chalked up four key achievements to the present time, all accomplished as a member of Prof. Zetian Mi’s research group:
- Demonstration of epitaxial growth and controllable doping in N-polar ultrawide-bandgap semiconductors.
- He demonstrated ScAlN based ferroelectric FETs with a large memory window using polarization-controlled conductance of an indium-tin oxide (ITO) channel.
- Demonstration of memory functionality in rare earth doped III-Nitride semiconductors epitaxially grown on GaN/sapphire templates by PAMBE, extending the family of nitride ferroelectrics for unique opportunities in III-nitride based memory devices for IoT applications.
- First demonstration of III-Nitride based high-performance self-powered reconfigurable photodetector based on epitaxially grown ferroelectric ScAlN thin film, operating in the deep UV spectrum.
Mondal was the principal author in the published research regarding each of these achievements, and received the APL Materials Excellence in Research Award in 2023 for the photodetector work (#4 above). Recently, he received a scholarship from the Society of Vacuum Coaters Foundation (SVCF) [read more].
“Shubham is not only an outstanding experimentalist, but also an exceptional researcher with a deep understanding of the underlying device physics and design,” said Prof. Mi. “Alongside his colleagues, Shubham has contributed significantly to groundbreaking advancements in the emerging fields of ultrawide bandgap III-nitrides and ferroelectrics.”
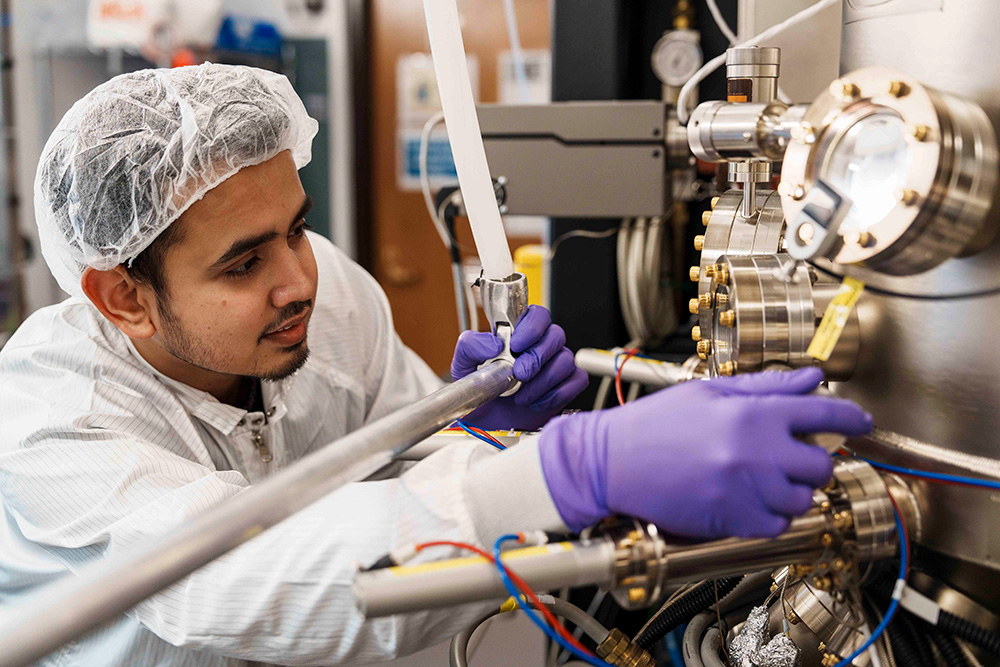
The research that Mondal does requires expensive, state-of-the-art equipment, including a Veeco GenXplor Plasma Assisted Molecular Beam Epitaxy (PAMBE) system and a suite of semiconductor equipment offered at the Lurie Nanofabrication Facility to fabricate devices. He then carries out a range of optical and structural characterizations in the LNF and the Michigan Center for Materials Characterization (MC)2.
Mondal received his undergraduate degree from Kalyani Government Engineering College in India. In addition to his PhD in ECE, he is pursuing a graduate certificate in Entrepreneurship and Innovation at the Center for Entrepreneurship, where he is applying his learning to help local businesses. Among these are Healing Not Hurting, a Black woman-owned holistic well-being business in Detroit, and A Brighter Way, a non-profit organization supporting formerly incarcerated individuals.
Mondal is a past President of the IEEE Electron Devices Society in India, and is currently a member of the engineering honor societies Tau Beta Pi and HKN. He is a member of the ECE Graduate Student Council, and co-led the BuddEEs program in 2021, which provided mentoring to incoming PhD students in ECE.